The Apple Watch 4 Is Giving Some Cardiologists Pause
The electrocardiogram capability of the Apple Watch Series 4 is a breakthrough, but some health experts worry it could lead to wasted time and money for doctors and patients.
CUPERTINO — A trio of flagship iPhones took center stage at Apple’s annual September event this week, as tradition has dictated for 11 years. But the redesigned Apple Watch Series 4 and its cutting-edge health alert features stole the spotlight.

The Series 4, which starts at $399 and goes on sale Sept. 21, has a a new electrical heart rate sensor that measures your heart’s rhythm in addition to the PPG (photoplethysmography) sensor that measures heart rate. Apple obtained clearance from the U.S. Food and Drug Administration for the device and its heart health apps, making it the only smartwatch on the market cleared to offer over-the-counter ECGs.
The technology could be game-changing for people who have heart conditions or risk factors for atrial fibrillation. But some cardiologists say the new ECG feature might send people running to the hospital for no reason. Should you be concerned? Here’s what you need to know.
How It Works

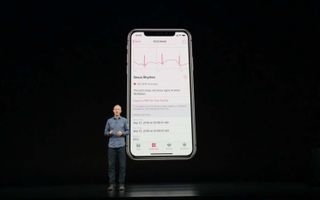
The Series 4 has a sapphire crystal back and a new Digital Crown with electrodes inside to diagnose atrial fibrillation (an irregular heart rhythm). Older Apple Watches running watchOS 4 can detect high heart rate and send alerts when BPM skyrockets outside of a workout. But that could be a sign of anything — AFib, perhaps, but also anxiety or even a pulmonary embolism. The Series 4’s ECG feature can actually diagnose atrial fibrillation.
MORE: Apple Watch Series 4 Hands-On: A Seriously Stunning Screen
To take a reading, you open the watch’s ECG app and press your fingertip to the Digital Crown for 30 seconds. Apple’s algorithm can detect whether your heart is in atrial fibrillation or sinus rhythm, which is normal heart rate activity. The results are stored in the iOS Health app, which you can share with your doctor.
Sign up to get the BEST of Tom's Guide direct to your inbox.
Get instant access to breaking news, the hottest reviews, great deals and helpful tips.
Atrial fibrillation is an irregular heart rhythm that can lead to life-threatening medical issues, including strokes. According to the Centers for Disease Control and Prevention, atrial fibrillation contributes to 130,000 deaths in the U.S. each year. People who have an irregular heart rhythm often don’t know they have it; there can be symptoms, but often you don’t feel any symptoms of AFib. That’s where the Apple Watch Series 4’s electrical heart rate sensor comes in. When paired with the ECG watch app, it could alert you about a heart condition you would have never known you had.
Why It’s So Major
The Series 4’s FDA clearance is meaningful, because it’s a Class II “de novo” clearance that signifies a first-of-its-kind device. In a statement issued on Twitter, FDA Commissioner Scott Gottlieb said the agency “worked closely with Apple as they developed and tested these apps, which may help many users identify health concerns more quickly.”
“Health care products on smartwatches may help users seek treatment earlier and will empower patients with more information about their health,” he added.
The American Heart Association also supports the Series 4’s new heart rate features. The organization’s president, Ivor Benjamin, appeared on-stage at the Apple event to talk up the Series 4’s life-saving potential.
“Getting FDA approval is by no means an insignificant step,” said IDC analyst Ramon Llamas. “The standards are extremely high and the margin for error is likewise slim, so congratulations are in order for Apple to get approval for the Series 4. Of course, if you look at the history of Apple’s partnerships and acquisitions, it was really only a matter of time for this to come together.”
Cause for Concern?
Ringing endorsements from reputable organizations aside, there are some downsides to a consumer device capable of diagnosing atrial fibrillation. False negatives are unlikely, but it’s possible the watch will diagnose AFib in people whose irregular heart rhythms could be attributable to a different, much less serious cause.
Dr. Gregory Marcus, director of clinical research for the University of California, San Francisco’s Division of Cardiology, said he’s seen patients who are unnecessarily alarmed by their heart rate data, costing doctors time and patients money that could have been used on something — or someone — else. That could get even worse with an easily accessible EKG.
MORE: The Doctor On Your Wrist: How Smartwatches Are Saving Lives
“The jury remains out as to whether there might be unanticipated harms from health care utilization, testing and subsequent intervention that occur due to false positives,” Marcus said. “Not to mention the potential unnecessary anxiety about what’s going on with the heart. Just with the PPG sensors in smartwatches and Fitbits, people are really concerned and come in to talk about that — their heart rate is too fast, too slow or changing. The only problem is they’re worried because of the device they’re wearing.”
Marcus leads UCSF’s Health eHeart Study, an ongoing clinical study that collects and examines heart rate data from a variety of wearable devices. (The study is always accepting new participants.) In November 2017, Apple launched its own Apple Heart Study in partnership with Stanford University’s School of Medicine to determine if the Apple Watch can accurately diagnose atrial fibrillation.
Apple submitted findings from two studies to the FDA as part of its submission to obtain clearance for the ECG app. According to STAT News, which obtained a summary of the findings from the FDA, the watch algorithm accurately identified 98.3 percent of people with atrial fibrillation, and correctly identified 99.6 percent of people who did not have atrial fibrillation. The study's sample size was 588 people.
Marcus isn't the only cardiologist who is concerned about the potential of false positives. In the day following Apple's announcement, other physicians in the industry reiterated his sentiments to CNBC, MarketWatch and Quartz. Marcus said he hopes Apple will follow up on its study and track the accuracy of the ECG app.
Who Really Needs An ECG Sensor?
Not everyone needs constant access to an at-home electrocardiogram. Marcus said people over 65 are at higher risk for atrial fibrillation and related heart conditions
“The elderly, people with hypertension, people with diabetes, heart failure and history of stroke would likely have the most to benefit by regularly checking this device for atrial fibrillation,” he said.
MORE: 3 Ways the Apple Watch Series 4 Can Save Your Life
That could be part of Apple’s strategy to make the Apple Watch more appealing to a wider variety of people. IDC analyst Llamas said the over-65 demographic is the least likely to buy a wearable device, but a child might buy one for an elderly parent to take advantage of the health features, including the EKG sensor and the Series 4’s new fall detection alert system.

“[Apple] understands how the watch works and how it keeps kids in contact with their aged parents, especially in case of emergency — see fall detection,” Llamas said. “Ultimately, these will end up on the wrists of older users, but while we’re at it, wouldn’t it be nice if the son or daughter gets a watch too?”
If the Series 4 sends people to the doctor more often, that might not be the worst thing in the world. But the watch isn’t a replacement for a doctor — even with an atrial fibrillation confirmation on the device, you should still seek out a professional opinion for diagnosis and treatment.
Photo credit: Apple
Caitlin is a Senior editor for Gizmodo. She has also worked on Tom's Guide, Macworld, PCWorld and the Las Vegas Review-Journal. When she's not testing out the latest devices, you can find her running around the streets of Los Angeles, putting in morning miles or searching for the best tacos.











-
photoew Watch it with calling people who are 65 elderly. 65 is the new 55. (Don't tell Congress!) Having had open hear surgery, and being diagnosed with AFIB the night before that surgery (thank goodness, I don't have to take blood thinners because they found it just in time) I'm a prime candidate for this. I've no illusions that it's as accurate as being hooked up in a hospital. But since I'm out and I don't have to visit my cardiologist for another five months, I like having this to just do a quick check occasionally. I know how to survey myself if that watch alerts me to something. So far, one alert since the surgery, and it was simply a blip and no need to get excited. I may not be typical. I've had nitro for over a year now and haven't had to use it once. But having this watch is going to make me worry a tiny bit less. And that's a good thing. UPS is here, my watch just arrived!Reply






